Our research
Our current research focuses can be divided into four segments:
- Enzyme Mechanisms,
- Enzyme Engineering and Biocatalysis,
- Metabolic Engineering and Synthetic Biology, and
- Bioreporters.
These research themes signify our aspiration to combine our discoveries and fundamental knowledge in enzymes and metabolic pathways with our mission to conduct research that supports developments in the areas of circular economy (CO2 utilization), biohydrogen production, green chemistry, food safety, biosensors and bioimaging.
Overview of current research in Chaiyen’s group

- Understanding enzyme reaction mechanisms
- Chemistry of the nature

- New ideas/new combination/ better efficiency
- Green and clean technology
- Challenging nature by creating new activities

- Constructing new pathways to do useful work
- Engineering enzymes to work better

- Detection/Detoxification/Biosynthesis
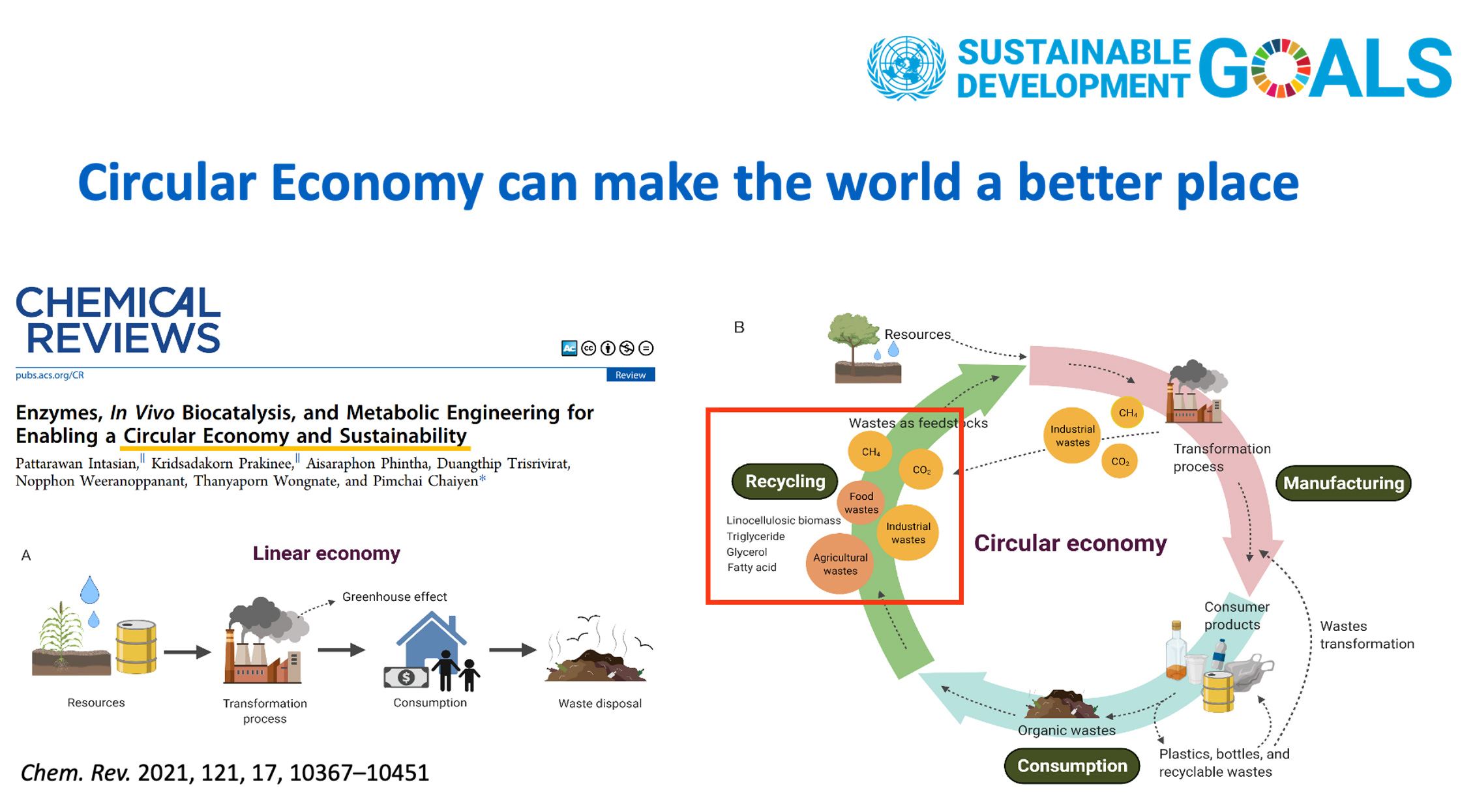
Chaiyen’s group – Supporting developments in the area of circular economy
Enzymes are catalysts that are vital for all organisms to maintain their cellular activities. Understanding enzyme reaction mechanisms has tremendous significance in technology development.
Our research group has long been recognized for pioneering work in the discovery of reaction mechanisms of flavin-dependent, redox and aldolase enzymes. We use a multi-disciplinary approach and a wide range of physical and biochemical tools and techniques such as transient kinetics, spectroscopy, structural biology and computational chemistry for studying enzymatic reactions.
Currently, our group studies reaction mechanisms of selected flavin-dependent monooxygenases, halogenases, dehalogenases and oxidases, electron transfer flavoenzymes, P450 enzymes and firefly luciferases. We have identified challenging questions and gap of knowledge in these enzymatic systems which should be tackled by mechanistic enzymology approach. Fundamental knowledge gained from studying these enzymatic systems will also be combined with development in application application proposes to obtain useful innovations in the future.

Picture illustrating diverse catalysis by a common C4a-(hydro)peroxyflavin in flavin-dependent monooxygenase.
(Reference: Phintha A. and Chaiyen P. Journal of Biological Chemistry, in press)
Selected recent publications
- Phintha A, Chaiyen P. Unifying and versatile features of flavin-dependent monooxygenases: diverse catalysis by a common C4a-(hydro)peroxyflavin. J Biol Chem, in press.
- Prakinee K, Phintha A, Visitsatthawong S, Lawan N, Sucharitakul J, Kantiwiriyawanitch C, Damborsky J, Chitnumsub P, van Pée KH, Chaiyen P. Mechanism-guided tunnel engineering to increase efciency of a favin-dependent halogenase. Nat Catal 2022 Jun;5:534-44.
- Phintha A, Prakinee K, Jaruwat A, Lawan N, Visitsatthawong S, Kantiwiriyawanitch C, Songsungthong W, Trisrivirat D, Chenprakhon P, Mulholland AJ, van Pee KH, Chitnumsub P, Chaiyen P. Dissecting the low catalytic capability of flavin-dependent halogenases. J Biol Chem 2021 Jan;296:100068.
- Pimviriyakul P, Jaruwat A, Chitnumsub P*, Chaiyen P*. Structural insights into a flavin-dependent dehalogenase HadA explain catalysis and substrate inhibition via quadruple π-stacking. J Biol Chem 2021 Aug;297(2):100952.
- Watthaisong P, Binlaeh A, Jaruwat A, Lawan N, Tantipisit J, Jaroensuk J, Chuaboon L, Phonbuppha J, Tinikul R, Chaiyen P, Chitnumsub P, Maenpuen S. Catalytic and structural insights into a stereospecific and thermostable Class II aldolase HpaI from Acinetobacter baumannii. J Biol Chem. 2021 Nov;291(5):101280.
- Trisrivirat D, Lawan N, Chenprakhon P, Matsui D, Asano Y, Chaiyen P*. Mechanistic insights into the dual activities of the single active site of L-lysine oxidase/monooxygenase from Pseudomonas sp. AIU 813. J Biol Chem. 2020 Aug 7;295(32):11246-11261.
- Pimviriyakul P, Surawatanawong P, Chaiyen P. Oxidative Dehalogenation and Denitration by a Flavin-dependent Monooxygenase is Controlled by Substrate Deprotonation. Chem Sci. 2018;9:7468-82.
Biocatalysis is the use of enzymes in synthesis of valuable chemicals. The chemicals industry is under pressure to conserve energy, reduce CO2 emissions and minimize toxic waste so as to reduce environmental damage. Biocatalysis is the use of enzymatic reactions in the forms of isolated enzymes or in whole cells to catalyze chemical reactions. The use of enzymatic reactions in chemical production can contribute to greener and cleaner industries. With the catalytic power of enzymes, processes can be operated under mild pH and temperature and without creation of hazardous intermediates and waste.
Our group leverages our in-depth understanding of enzymatic mechanisms to improve enzymatic properties via enzyme engineering approach. We are well equipped with robotic high-throughput liquid handing and high-resolution mass spectrometry systems which allow us to perform cutting-edge enzyme engineering work. This semi-rational or mechanism-guided approach has resulted in successful development of efficient and robust enzymes and biocatalysts that meet industrial standards.
Currently, our group studies engineering and biocatalysis of several flavin-dependent, P450 and other redox enzymes. These enzymes are attractive for green chemistry applications such as for synthesis active pharmaceutical ingredients or valuable bioactive compounds. Biocatalytic reactions are considered as green technology for the future because the process does not require high energy, harsh conditions or toxic chemicals.
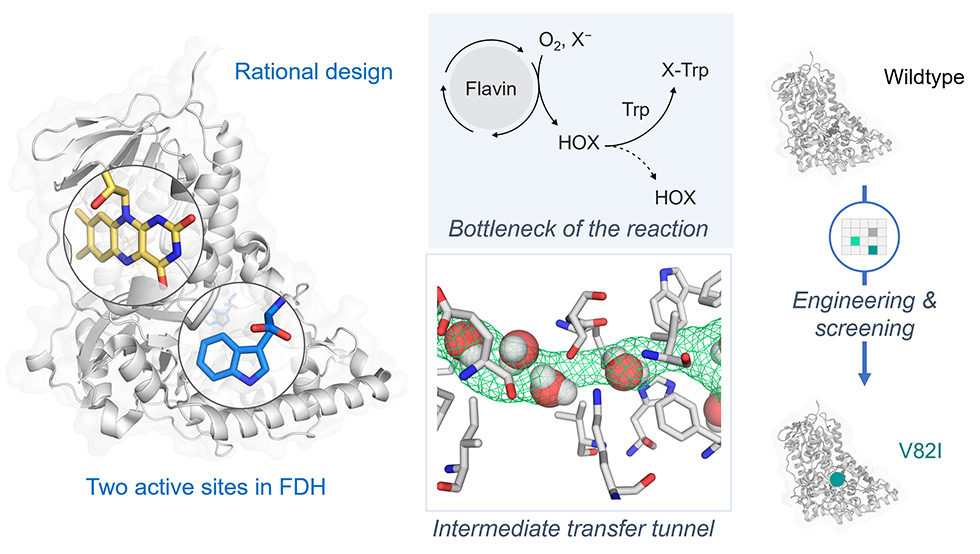
Use of mechanism-guided approach to improve catalytic properties of a flavin-dependent halogenase
(Reference: Prakinee K, Phintha A, et al. Nat Catal 2022 Jun;5:534-44)
Selected recent publications
- Prakinee K, Phintha A, Visitsatthawong S, Lawan N, Sucharitakul J, Kantiwiriyawanitch C, Damborsky J, Chitnumsub P, van Pée KH, Chaiyen P. Mechanism-guided tunnel engineering to increase efciency of a favin-dependent halogenase. Nat Catal 2022 Jun;5:534-44.
- Phintha A, Chaiyen P. Rational and mechanistic approaches for improving biocatalyst performance. Chem Catalysis 2022;2(10):2614-2643.
- Watthaisong P, Kamutira P, Kesornpun C, Pongsupasa V, Phonbuppha J, Tinikul R, Maenpuen S, Wongnate T, Nishihara R, Ohmiya Y, Chaiyen P. Luciferin Synthesis and Pesticide Detection by Luminescence Enzymatic Cascades. Angew Chem Int Ed Engl 2022 Apr;61(16):e202116908.
- Pongpamorn P, Kiattisewee C, Kittipanukul N, Jaroensuk J, Trisrivirat D, Maenpuen S, and Chaiyen P. Carboxylic Acid Reductase Can Catalyze Ester Synthesis in Aqueous Environments. Angew Chem Int Ed Engl 2021 Mar;60(11):5749-53.
- Watthaisong P, Pongpamorn P, Pimviriyakul P, Maenpuen S, Ohmiya Y, Chaiyen P. A Chemo-Enzymatic Cascade for the Smart Detection of Nitro- and Halogenated Phenols. Angew Chem Int Ed Engl. 2019 Sep;58(38):13254-13258.
- Chuaboon L, Wongnate T, Punthong P, Kiattisewee C, Lawan N, Hsu CY, Lin CH, Bornscheuer U, Chaiyen P. One-Pot Bioconversion of L-Arabinose to L-Ribulose in an Enzymatic Cascade. Angew Chem Int Ed Engl. 2019 Feb 18;58(8):2428-2432.
Synthetic biology is the design and construction of new biological entities such as enzymes, genetic circuits, and cells or the redesign of existing biological systems. Research in this field is multidisciplinary by nature and employs an engineering approach to construct and put together new or engineered biomolecular units such enzymes, genetic circuits, metabolic pathways, etc. In general, the assembly of these biomolecular parts and devices is performed to solve specific problems or accomplish specific goals.
Synthetic biology projects in our group employ state-of-the-art technology to construct engineered cells and new metabolic pathways via metabolic engineering approach for turning low value feedstock such as sugar, CO2 and bio-wastes such as lignocellulosic biomass and triglyceride into value-added products. Recently, we have developed a promising cell prototype which can significantly enhance the amount of biohydrogen production. We are collaborating with leading industrial partners to develop cell prototypes which can be used in industrial scale production of valuable compounds from low value feedstocks. We believe that technology development in this research area and partnership with private sector are important for making industrial processes more sustainable and mitigating environmental damage and climate change.

Current work theme in Synthetic Biology area in Chaiyen’s group.
Selected recent publications
- Intasian P, Sutthaphirom C, Binlaeh A, Phonbuppha J, Jaroensuk J, Teanphonkrang S, Woraruthai T, Tirapanampai C, Onchan W, Schulte A, Buckel W, Weeranoppanant N, Wongnate T, Sucharitakul J, Chaiyen P. Empowering extra fuel supply in E. coli by electron bifurcation for robust H2, ATP and succinate production. ChemRxiv. Cambridge: Cambridge Open Engage; 2023
- Joroensuk J, Sutthaphirom C, Phonbuppha J, Chinantuya W, Kesornpun C, Akeratchatapan N, Kittipanukul N, Phatinuwat K, Atichartpongkul S, Fuangthong M, Pongtharangkul T. A versatile in situ cofactor enhancing system for meeting cellular demands for engineered metabolic pathways. bioRxiv. 2023:2023-01.
- Trisrivirat D, Tinikul R, Chaiyen P. Synthetic microbes and biocatalyst designs in Thailand. Biotechnol Notes 2023;4:28-40.
- Intasian P, Prakinee K, Phintha A, Trisrivirat D, Weeranoppanant N, Wongnate T, Chaiyen P*. Enzymes, In Vivo Biocatalysis, and Metabolic Engineering for Enabling a Circular Economy and Sustainability. Chem Rev 2021;121(17):10367-10451.
- Jaroensuk J, Intasian P, Kiattisewee C, Munkajohnpon P, Chunthaboon P, Buttranon S, Trisrivirat D, Wongnate T, Maenpuen S, Tinikul R, Chaiyen P. Addition of formate dehydrogenase increases the production of renewable alkane from an engineered metabolic pathway. J Biol Chem. 2019 Jul 26;294(30):11536-11548.
- Jaroensuk J, Intasian P, Wattanasuepsin W, Akeratchatapan N, Kesornpun C, Kittipanukul N, Chaiyen P. Enzymatic reactions and pathway engineering for the production of renewable hydrocarbons. J Biotechnol. 2020 Feb 10;309:1-19.
Nature is smart and beautiful, and we harness its power in finding elegant solutions to practical problems. Some of the enzymatic reactions studied by our group can generate signals such as light or electrical output. These reactions can be linked to detection of specific molecules including genes related to diseases or pesticide contaminants in the environment and food produce.
We have developed bioreporting technologies based on enzymatic reactions of luciferases or enzymes that can emit blue, green or orange light. These reactions can be used as in vivo cell monitoring tools for specific gene or disease detection in experimental animals. Our group also has developed a new enzymatic cascade reaction for the synthesis of luciferin analogs from phenolic compounds, (HELP reaction). Luciferin is the substrate of the enzyme luciferase and causes fireflies to glow. Bioluminescence catalyzed by luciferase is an important method used in biochemical research and the analysis of toxitoxicantsns. HELP makes it possible to produce luciferin analogs much more easily than before, without special expertise or toxic chemicals. Our team also produced two novel luciferin analogs. One of these produces brighter light of a longer wavelength than the original, which penetrates cells and tissues more efficiently. This facilitates real-time imaging and helps to reduce animal experiments.
Our group has also invented a proprietary, enzymatic reaction-based method named LUMOS (LUminescence-related Measurement Of Specific Detection) to detect and degrade pesticides in one-pot system. The overuse of toxic organophosphate pesticides (OPs) is a serious global environmental and health problem. It affects farmers who work the toxic land and consumers who eat the toxic produce. Toxic pesticide residues also contaminate ground water and water reservoirs, and have been linked to cancer, Alzheimer’s, and diabetes. LUMOS works in three reaction steps:
- Pesticide degrading enzymes break down OPs and/or their metabolites into phenol derivatives.
- HELP reaction converts phenolic derivatives to a luciferin analog.
- Addition of firefly luciferase generates a bioluminescence signal. The wavelength is used to differentiate between different OPs. Our team has been able to detect five particularly toxic OPs, such as parathion (E605), in concentrations of parts per trillion – onsite and without sample preparation - in urine, blood serum, and fruit.

LUMOS technology can degrade pesticides and convert them into luciferin derivatives. By adding the enzyme luciferase, the systems generate light emission for pesticide detection.
References: Watthaisong P et al. Angew Chem Int Ed Engl. 2019 Sep;58(38):13254-13258.
Watthaisong P et al. Angew Chem Int Ed Engl 2022 Apr;61(16):e202116908.
Selected recent publications
- Phonbuppha J, Tinikul R, Ohmiya Y, Chaiyen P. High sensitivity and low-cost flavin luciferase (FLUXVc)-based reporter gene for mammalian cell expression. J Biol Chem 2023 May;299(5):104639.
- Watthaisong P, Kamutira P, Kesornpun C, Pongsupasa V, Phonbuppha J, Tinikul R, Maenpuen S, Wongnate T, Nishihara R, Ohmiya Y, Chaiyen P. Luciferin Synthesis and Pesticide Detection by Luminescence Enzymatic Cascades. Angew Chem Int Ed Engl 2022 Apr;61(16):e202116908.
- Tinikul R, Trisrivirat D, Chinantuya W, Wongnate T, Watthaisong P, Phonbuppha J, Chaiyen P*. Detection of cellular metabolites by redox enzymatic cascades. Biotechnol J 2022 Jun;17(6):2100466.
- Phonbuppha J, Tinikul R, Wongnate T, Intasian P, Hollmann F, Paul CE, Chaiyen P. A Minimized Chemoenzymatic Cascade for Bacterial Luciferase in Bioreporter Applications. Chembiochem 2020 Jul;21(14):2073-79.
- Watthaisong P, Pongpamorn P, Pimviriyakul P, Maenpuen S, Ohmiya Y, Chaiyen P. A Chemo-Enzymatic Cascade for the Smart Detection of Nitro- and Halogenated Phenols. Angew Chem Int Ed Engl. 2019 Sep;58(38):13254-13258.
- Highlighted as "Hot Paper:Biocatalysis" and "Frontispiece Article"
- Highlighted in more than 8 news outlets- Chem Europe on 13 Aug 2019
- Bionity on 13 Aug 2019
- Azcom.com on 12 Aug 2019
- The Meical News on 12 Aug 2019
- Technology Networks on 9 Aug 2019
- Science Daily on 8 Aug 2019
- ChemistryViews on 8 Aug 2019
- Phys.org on 8 Aug 2019